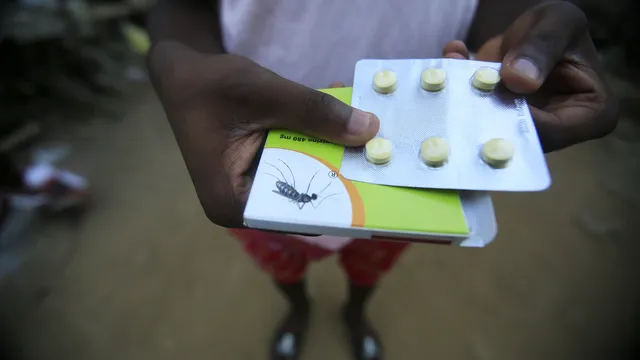
The first malaria drug for newborns and infants has been approved by Swiss health regulators. Eight African countries are ready to launch the drug on the market in the near future. This was announced by pharmaceutical giant Novartis.
Malaria is a major cause of death in Africa. According to the World Health Organization (WHO), in 2023, 95% of the 597,000 deaths from this disease worldwide occurred on this continent.
According to the WHO, about 76% of these deaths—more than 432,000—were children under the age of 5, AFP reported.
The infant version of Novartis' Coartem drug, also known as Riamet, was developed in collaboration with Medicines for Malaria Venture (MMV), a Geneva-based organization specializing in research and development of drugs to combat the disease.
“Until now, there has been no approved treatment for malaria in infants weighing less than 4.5 kilograms,” Novartis said.
The approval from the Swiss health authorities is for a dose intended for babies weighing between 2 and 5 kilograms.
Eight of the countries most affected by malaria—Burkina Faso, Côte d'Ivoire, Kenya, Malawi, Mozambique, Nigeria, Tanzania, and Uganda—participated in the Swiss approval process.
These countries are now expected to quickly approve the treatment under a program to facilitate access to medicines for low- and middle-income countries.
The Swiss pharmaceutical company said it would introduce the treatment “on a non-profit basis” to increase access in places where this mosquito-borne disease is endemic.
According to the WHO, around 263 million people worldwide were infected with malaria in 2023.
Public health experts say funding to combat the disease is at risk following US President Donald Trump's decisions to cut foreign aid.
Previously, the US government provided about 40% of annual global funding for malaria control and research. | BGNES

 Breaking news
Breaking news
 Europe
Europe
 Bulgaria
Bulgaria